Many people don’t know, but in Europe, personal lubricants are classified as medical devices, and are as strictly regulated as defibrillators or infusion pumps, for example. Next year even stricter rules come into force for personal lubricant manufacturers – they will no longer be allowed to sell personal lubricants if they do not have certain certifications. Their products will only be classified as cosmetics. But what exactly is the difference? We explain what you need to look out for the next time you buy a personal lubricant.
- What is a medical device?
- Classification of medical devices
- And what does the word “cosmetic” refer to?
- Information requirement on cosmetic products
- Why is it important to know the difference?
What is a medical device?
Let’s start off with some theory. 🤓
In general, a medical device refers to any instrument, apparatus, machine, software, implant, reagent, material, or other similar article intended by the manufacturer to be used with humans alone or in combination for one or more of the following specific purposes:
- Diagnosis
- Contraception
- Monitoring
- Projection
- Prognosis
Products for contraception or conception are also classified as medical devices. Medical devices are subject to separate regulations – depending on the risk class.
Classification of medical devices
Medical devices are subdivided into four risk classes. This classification depends on the area of use, duration of use, anatomic position, and the resulting risk linked to use. The classification reaches from no risk present through high risk and potential risk.
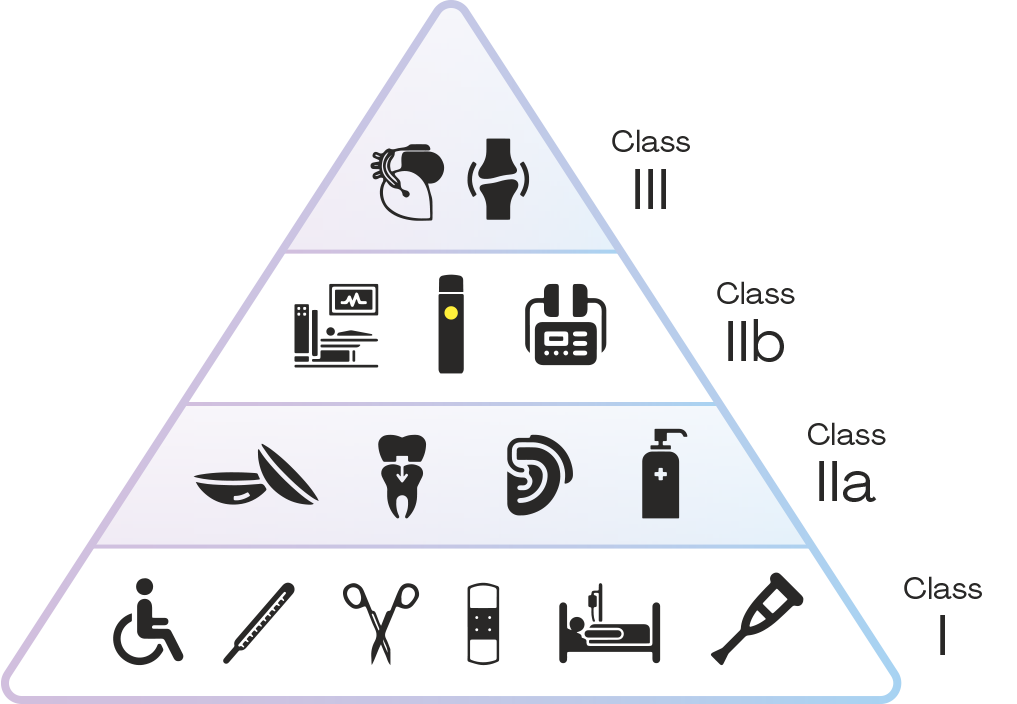
Devices in Class I are low risk and do not require certification by an official body. This includes reading glasses and support stockings, for example. Class I is also divided into sterile medical devices (Class Is) and medical devices with a measuring function (Class Im).
Then come Class II medical devices, which are then subdivided into IIa and IIb. Products in Class IIa have a medium and Class IIb a greater risk. To obtain the CE mark for devices in both Classes and bring them onto the market, manufacturers require an EU declaration of conformity, or “CE certificate.” Devices in Class IIa include disposable syringes or ultrasound scanners.
Devices in Class IIb are partly or completely introduced into the body. They come with a greater risk than IIa products and devices. This includes infusion pumps, ventilators, defibrillators, and personal lubricants and condoms. Then comes Class III, which covers products and devices that are applied directly to the central nervous or cardiovascular system, for example pacemakers or prosthetic heart valves.
When you realize the impacts that Class IIb devices have on the body and the risk potential ascribed to them by the MDR (European Medical Device Regulation), you will understand the importance of regulating personal lubricants. Remember: personal lubricants are introduced into the body and therefore must meet the highest standards to ensure users’ safety. So, always make sure you buy a certified personal lubricant. To be sure, check the information on the label.
And what does the word “cosmetic” refer to?
Cosmetic covers substances that are exclusively for external use – on the skin, nails, hair, and teeth. The purpose of these products must be to:
- Clean,
- Perfume,
- Change the appearance,
- Protect, or
- Affect the body’s smell.

Information requirement on cosmetic products
Just because cosmetics are intended for external use doesn’t mean that they are not also strictly controlled and regulated. Here too, the safety of the user must be guaranteed. That is why cosmetic products must include certain information on the label. Here’s a brief overview of the most important information:
Cosmetics need to identify their ingredients on the label, just like packaged food, so that consumers can trace whether they contain critical substances or not. The EU Cosmetics Law specifies a certain order in which all ingredients are listed. The ingredients are listed by their proportionate weight in decreasing order. The more of a substance is contained, the higher up it is in the list. This applies to all ingredients that make up more than one percent of the content. But watch out: odorants and flavorings do not have to be listed separately. They can be summarized – for example using the word “perfume” or “aroma.” However, 26 fragrances that most frequently trigger allergies in humans across Europe are excluded from this rule. These fragrances must be identified.
Cosmetics must have a best-before date. This can be identified either by the sentence “best before” or by a certain symbol (egg timer with date). The best-before date tells you that, if the product is stored correctly, it will still have its original quality and function. However, the product can still be used without risk after the best-before date has expired – because the best-before date is not a use-by date!
For cosmetics that last for longer than 30 months, other claims apply. Here the symbol of an opened jar of cream must be printed, with its shelf life once opened.
It is easy to tell whether a product is no longer any good: smell the product. If it smells rancid, chuck it in the trash. Or if the components have separated, then it’s better to throw it away.
Why is it important to know the difference?
Most of you might think this information is nice to know – but has no actual purpose. Well, you’d be wrong!
To start with: safety. Because personal lubricants are introduced into the body, they are classified as medical devices and are therefore as strictly regulated and controlled as defibrillators. From next year, personal lubricant manufacturers must also be certified under the MDR (European Medical Device Regulation) if they want to continue to manufacture and sell products under the name of personal lubricants or gels. Only these certified products meet the high requirements for a Class IIb medical device and guarantee safety for your body.
With cosmetics or food, most of us pay attention to the ingredients, test approvals, or origin of the components and other claims. We really value quality. Why shouldn’t it be the same for personal lubricants? Because: only the best for you and your body!
Image sources: pexels-cottonbro-studio-8666804, pexels-koolshooters-8946864
